Self-Healing Soft Pneumatic Robotics
It is a long-standing interest of the robotics community to investigate actuators with performances matching or exceeding the ones of biological muscles. Currently, the majority of robots are still powered by stiff actuators, which do not exploit the unique soft properties of the muscle-tendon system. However, compliant/soft robotic features are promising for locomotion, manipulation or wearable robotics to reach expected performances and safety during interaction with humans or uncertain and dynamic environments.
Currently, a large class in soft robotics consist of soft pneumatic actuators (SPA), which are mostly constructed (almost) entirely out of soft, (hyper) elastic materials (Ecoflex®) and are, like the name indicates, actuated by compressed air. Because of these intrinic softness, SPAs have the ability to resist mechanical impacts that would irreversibly damage or destroy hard robotic systems. This is a very interesting feature, since these actuators will be used in next generation (soft) robots, which will function in unknown, unstructured and non-predefined environments; in other words, the world we are living in. They will be subjected to unexpected damaging conditions, such as impacts and collisions. The mechanical softness of the SPAs will protect them against these hostile events.
However, the use of SPAs introduces a new problem: the actuator parts can be easily ripped, perforated or scratched by sharp objects presented in hostile environments.
This problem can be solved by designing SPAs and compliant elements entirely out of self-healing (SH) polymers. Up till now, the self-healing material technology is not yet explored in robotics. Recent development in SH-polymers have led to (commercial) applications and made us investigate the potential of using these materials in robotics.
Self-healing materials
Natural organisms have a remarkable, unique property, the ability to self-heal when certain damages and injuries occur. Their bodies are not over-dimensioned and fractures, ruptures and injuries will occur when a body part is overloaded in abnormal, extreme circumstances. The powerful biological healing function has inspired chemists to impart similar properties to synthetic materials to create “self-healing materials”. Since 2001, a broad range of self-healing (SH) materials has been developed. Self-healing mechanisms have been developed for metals and ceramics, but self-healing polymers showed laterly the largest evolution. Recent developments in the self-healing polymer technology have led to (commercial) applications.
Definition of self-healing material
There has been some discussions on the definition of a SH-material and therefore sometimes other denominations are used; eg. remendible materials. We follow a rather general definition; "Self-healing materials are polymers, metals, ceramics and their composites that when damaged through thermal, mechanical, ballistic or other means have the ability to heal and restore the material to its original set of properties." The healing procedure can be either spontaneous or take place with aid of a stimulus. Few materials intrinsically posses this ability.
Overview self-healing polymers
Because we introduced a self-healing mechanism in the soft robotics through the use of a SH-polymer we focus our research on polymers. Although SH-metal and ceramic applications exist, self-healing polymers recently have made the fastest evolution. In this section, a brief classification of SH-polymers is presented.
 |
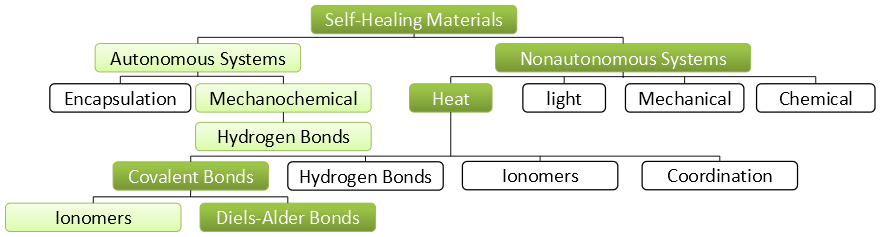
First two distinct classes can be dened: autonomic self-healing materials and non-autonomic systems. Autonomic systems require no stimulus (other than the formation of damage) for operation. These mechanisms do not require human intervention and are entirely self-contained. They most closely resemble biological systems, which deliver healing agents to compromised regions as soon as damage is inflicted. Non-autonomic systems on the other hand require some type of externally applied stimulus (such as heat or light) to enable a healing function. Yet this allows the healing process to be performed in a controlled way.
Autonomous self-healing polymers
Encapsulation
A subcategory of autonomic systems are the self-healing mechanisms using encapsulation. In this case, reactive chemical reagents (so called healing agents) are stored through compartmentalization, usually in the form of microcapsules, inside the material. When damage is occurs, the capsules will break and the chemicals are released and interact in such a manner that void spaces become filled, and through a chemical reaction the surfaces bond together.
Application in soft-robotics: A disadvantage of systems working with a form of encapsulated reagents is that after the healing process the material properties will change locally. An even greater disadvantage is that the healing process can only operate once (or a limited number of times) at the same location because once the capsules are broken the healing mechanism is no longer present at that specific location. This means that the number of damage-healing cycles is strongly limited. Due to these two drawbacks this encapsulation self-healing system is not suitable for soft robotic applications. However encapsulation can be interesting for surface applications in (hard) robotics to protect, for example, the cover/skin against appearing scratch damages, which usually do not take place at the exact same location. New self-healing polymers are beeing developed which provide a solution to these drawbacks through the use of a vascular network instead of a discrete capsulation. However, this vascular network is yet too complex to be introduced in soft robotics.
Mechanochemical
Other autonomic systems are joined under the name: mechanochemical systems. These mechanisms rely on weak reversible interactions (weak metal-amine bonds or weak hydrogen bonds), in conjunction with a polymeric structure designed to bear and later release stress. These weak reversible interactions (which form a reversible network by cross-linking) will break under a sufficient mechanical stress. But in unstressed condition at ambient temperature, the bonds will be reconstructed again due to the reversible behavior of the reaction, regenerating the chemical bond, without the need of an external stimulus.
Application in soft-robotics: The mechanical properties of these polymers can be affected by the composition and the concentration of the weak bonds in the polymer structure. Up till now, mechanochemical polymers with mechanical properties adequate for soft robotics (high strength, low visco-elastic behavior,...) cannot be synthesized, due to the presents weak bonds, of which the reversible network is formed. However, these materials are very promising, since the ability to perform self-healing at ambient temperature, without an external stimulus or encapsulation, is a huge advantage in comparison with the other non-autonomous self-healing mechanisms.
Non-autonomous self-healing polymers
As already mentioned non-autonomic self-healing materials always require some type of external stimulus. This stimulus can be in the form of heat, light, a mechanical or chemical stimulus.
light
Light can be used to induce chemical based healing processes. An advantage of this mechanism is that photoreactions are usually very fast and can be selectively initiated by applying light of an appropriate wavelength (visible light or UV-light). Light also enables greater control over the healing process since it can be localized to specific sites.
Application in soft-robotics: A big drawback is that the healing process is usually only limited to the surface areas only, because of the limited penetration depth of the light, due to its absorption. Hence bulk or macroscopic healing is not possible photo-induced self-healing polymers are less interesting for their implementation in soft robotics. However, they show a very high potential for future coating applications in (hard) robotics.
Heat
Heat is used in most of the self-healing polymers already developed. Compared with the other non-autonomic self-healing systems, these mechanisms are most applicable in soft robotics design, because of their relatively easy thermal control. Based on their chemistry they can be classied as follows.
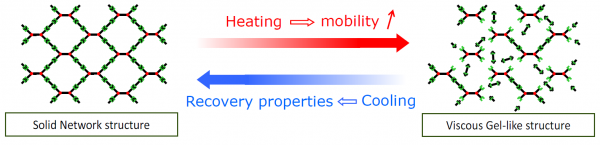
At room temperature, all these polymers consist of a thermo-reversible network, which is build up by cross-linking of thermo-reversible bonds. If heat is applied and the polymers are brought to elavated temperature these cross-linking bonds are reversibly broken. The network is broken (the polymer has an almost gel-like consistance) and polymer chains obtain enough mobility to slowly fill/seal/close microscopic and macroscopic damages, such as gaps, cuts, tears, cracks, perforations and punctures. After these damages are cured, the polymer is cooled down to room temperature and the reversible network is formed again. There exist already a broad number of thermo-reversible network polymers which rely on different chemical cross-link bonds; eg. covalent bond SH-polymers, coordination bond SH-polymers, hydrogen SH-polymers and Ionomers.
Application in soft robotics: Because there are a lot of different thermoreversible SH-polymers available, their differences are further elaborated in the next paragraphs.
Thermo-reversible self-healing polymers
Covalent bond
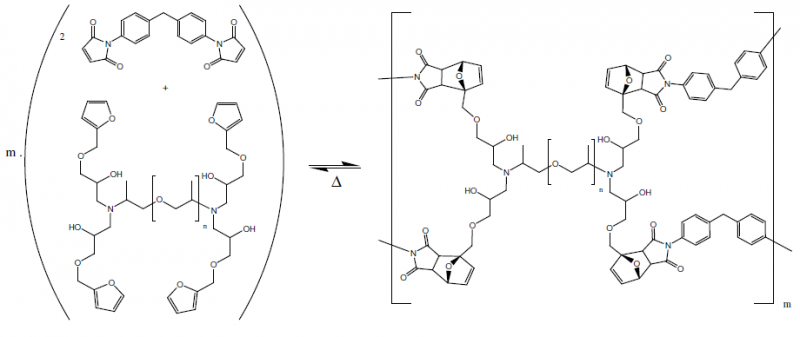
Diels-Alder polymers are the most commenly used. Their thermo-reversible network is formed by a Diels-Alder (DA) cross-linking reaction between a furan ring and a maleimide ring.
Application in soft-robotics: Because their cross-linking is based on covalent bonds, the DA-polymers and covalent bond SH-polymers in general, posses engineering mechanical properties (high fracture strain and high ultimate tensile strength). In theory, there mechanical properties are almost completely recovered after healing, which means that the number of SH-cycles for a polymer part is not limited by the SH-mechanism. The non-autonomous self-healing process requires temperatures in the range of 70 to 120 °C.
Coordination bond
Coordination bond polymers are composed of monomers that interact non-covalently. Common interactions include metal-ligand coordination, and van der Waals forces. Mechanical stress in these polymers causes the disruption of these specific non-covalent interactions, leading to monomer separation and polymer breakdown. These weak interactions are of particular interest for self-healing materials because of their reversible nature.
Application in soft-robotics: Because their network relies on non-covalent (weak) bonds, these materials lack good mechanical properties, a lot of them have a gelatinous behavior at ambient temperature. This disadvantage make coordination bond polymers less suitable for self-healing actuator application.
Hydrogen bond
Like coordination bond SH-polymers, the thermo-reversible network relies on non-covalent bonds; hydrogen bonds.
Application in soft-robotics: Although the heat required for their SH-process is much lower than the one required for covalent bond SH-polymers, these H-bond SH-polymers lack adequate mechanical properties.
Ionomers
Ionomers are a class of polymers that contain as much as 20% of charged or ionic species as a part of their structures. These ions are able to interact with one another in a manner very similar to salt bridges seen in proteins, creating interactions or aggregates that have a profound effect upon their mechanical and physical properties. These aggregates consist of several ion pairs known as multipelts. When stressed, microscopic or macroscopic damages can occur in the material because these multipelts are broken. The non-autonomous healing process of these materials relies again on a heat stimulus: As heating occurs, whether through direct application or as a result of the friction resulting from damage, the multiplets dissociate (the thermo-reversible network is dissociated). This gives the material enough mobility to seal and close the micro/macroscopic damages. Cooling reverses the processes and the network is formed again. However, the material does not regain its native structure until the multiplets re-form, which can take days.
Application in soft-robotics: Because of their crystalline structure, SH-ionomers can be synthesized, having mechanical properties which are adequate for self-healing soft robotics. Their mechanical properties can be partially recovered after a SH-process, or fully if waited long enough. The non-autonomous self-healing process requires temperatures in the range of 110°C to 150 °C.
Although classied as non-autonomous, the damage event can provide enough energy, generally in the form of heat as a result of friction, so that the material eectively behaves as an autonomic self-healing material. This is what happens if the self-healing material is used for healing of ballistic impacts, the application where these polymers are used mainly. For example there exist already ionomer plates, which remain fully intact after a bullet impact. The bullet will penetrate the polymer and due to the heat induced by friction the hole, left behind by the bullet, will be closed immediately. These "Impact Seal Self-Healing Targets" are being commercialized and used as long lifetime targets on shooting ranges
Current applications of self-healing materials
Self-healing coating of LG flex 2

Scratch shield paint of Nissan
Self-healing asphalt of Heijmans

Self-healing paint for aerplanes of KLM-Air France
Puncture SH-polymers for targets and aerospace

Self-healing materials in soft robotics
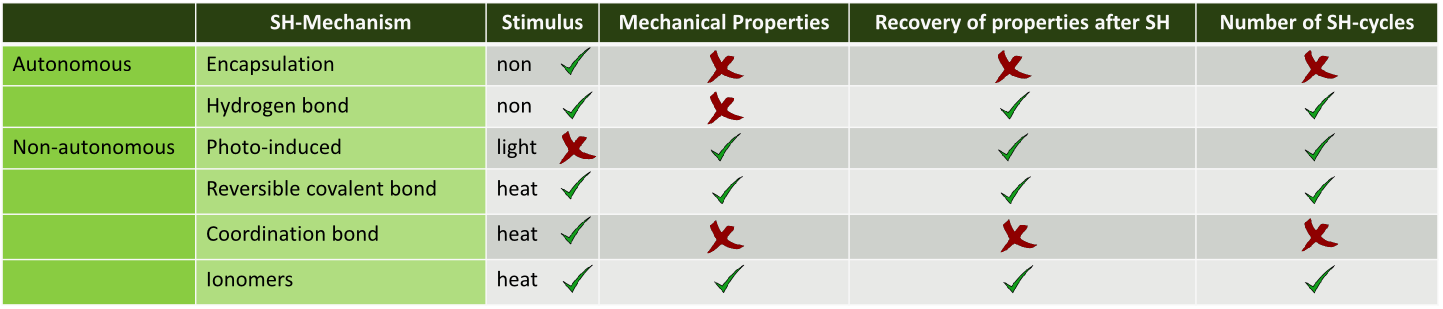
Although a wide range of SH-polymers has been developed over the last years, only few have properties that comply with the specific requirements for soft pneumatic robotics. This was concluded from an analysis (table) which was conducted on the SH-mechanisms, presented in figure.
From this analysis, the DA- SH-polymers were chosen for the construction of the first SH-SPA. The SH-process of these non-autonomous DA-polymers requires a heat stimulus and is based on reversible covalent bonds. One of the reasons why the DA-polymers were chosen is because the mechanical properties of these polymers are almost completely recovered after healing, which means that the number of SH-cycles for a polymer part is not limited by the SH-mechanism. This is not the case for SH-mechanisms using coordination bonds or encapsulation mechanisms. In case of an encapsulated system the healing action can only take place a limited number of times at the same damage location: once the capsules are broken the healing agent is no longer present at that specific location, which strongly limits the number of damage-healing cycles.
A second advantage of the DA-polymers are the strong reversible bonds the network consists of. Due to these, the polymers have a relatively high ultimate tensile strength, as required for SPA-applications. The SH-process of both coordination bond and mechanochemical mechanisms, on the other hand, is based on weak non-covalent interactions, such as hydrogen bonds or weak metal-amine bonds, limiting the mechanical properties of these polymers.
Similar to the DA-polymers, SH-ionomers, in which the SH-process also requires a heat stimulus, show a good recovery of the initial mechanical properties after healing, as well as adequate mechanical properties. However, the DA-polymers were selected because their SH-can take place at temperatures as low as 70 °C, which is clearly lower than the required temperature for the SH-process of the ionomers, which is 110 °C or higher.
The photo-induced healing of polymers requires a light stimulus with a specific frequency and of sufficient intensity. The network of these polymers is built up by cross-links based on photo-reversible bonds. These covalent cross-links provide adequate mechanical properties, comparable to the reversible DA-cross- links. Due to the limited penetration depth of the light, the SH-process can only occur in relatively thin polymer pieces (1–2 mm), which might be deep enough for some soft pneumatic robotics appliactions. As currently intense UV-light is needed to heal these polymers, these photo-induced polymers are not used in this research work. Nevertheless photo-induced SH-polymers have potential to be used in future Soft Robotics applications.
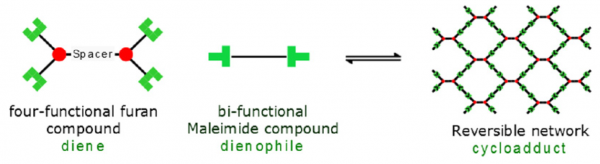
Diels-Alder Polymers
The DA-polymer is a thermoreversible polymer network formed by a DA-cross-linking reaction, between a furan rings, present on the synthesized four-functional furan compound, with the maleimide rings, present on the bi-functional maleimide compound.
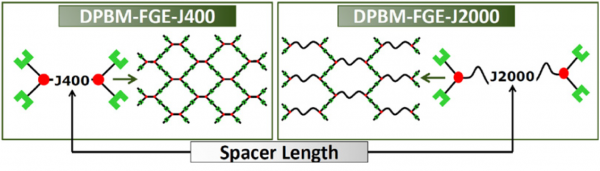
The synthesis allows tuning of the mechanical properties of the DA-polymers by varying the furan spacer length, which is the chain length of the poly(propylene oxide) chain of the Jeffamine used. A series of three DA-polymers, noted DPBM-FGE-J400, -J2000 and -J4000, with diverse mechanical properties was synthesized.
Properties of Diels-Alder polymers
Classification of DA-polymers
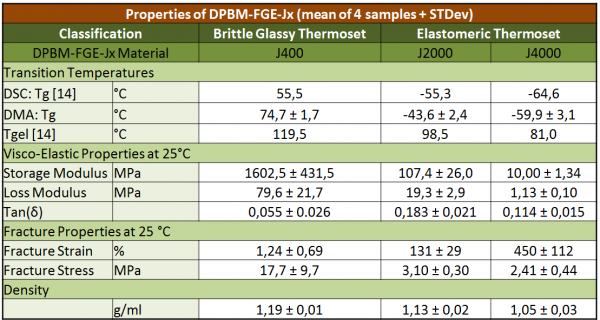
The three DA-materials, -J400, -J2000 and -J4000, can be classified in two groups: the ‘reversible glassy thermosets’ and the ‘reversible elastomeric thermosets’, based on their viscoelastic behavior at ambient temperature (Tambient).
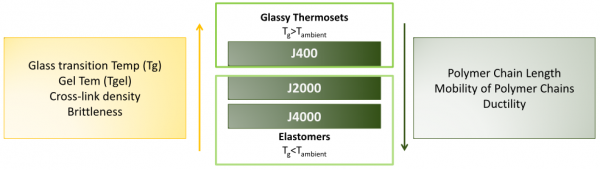
To explain this classification, the glass transition temperature (Tg) has to be introduced. Tg is the temperature at which an amorphous material undergoes a reversible transition from a hard and relatively brittle glassy state into a molten or rubbery state (upon heating). On the one hand, -J400 contains furan compounds with short polymer chain length. Due to these short chains, the network has a high (reversible) crosslink density, which raises the Tg of the polymer above Tambient, leading to (brittle) glassy thermosets at Tambient. -J2000 and -J4000 on the other hand are built up out of furan compounds with longer polymer chain length, limiting the cross-link density. The latter materials are elastomeric thermosets, with ductile characteristics, and having a Tg lower then Tambient. In the table, the Tg, and the densities of the three DA-polymer materials are presented.
SPAs are constructed from hyperelastic polymers. Therefore, both elastomer DA-materials, -J2000 and -J4000, are combined in the construction of a first SH-soft pneumatic actuator (SH-SPA), exploiting the difference in mechanical properties of these two materials in the mechanical design of the SH-SPA. The brittle, -J400 material cannot be used in SPAs, however, it can be used in other robotic applications.
Recently, we used this brittle -J400 material to develop a self-healing mechanical fuse (SH-MF), which can be integrated in a series elastic actuator. This SH-MF protects the system from damaging overloads. Upon an overload on the system, potentially damaging one of the actuator components, the fuse fractures sacrificially and will be healed after removal of the overload. Using this principle all components are protected and there is no need for large over-dimensioning.
Mechanical properties of the DA-series
In order to determine the viscoelastic behavior of the self-healing polymer series at ambient temperature, Dynamic Mechanical Analysis (DMA) was carried out on the three materials. In the table the Storage modulus, Loss modulus and their ratio, the tan(delta) are presented. At 25 °C -J400 is in the glassy region (Table I), where the storage modulus (2280 MPa) is high compared to the loss modulus (111 MPa), which results in a low tan(delta), indicating that there is almost no viscous contribution. -J400 behaves like an elastic solid, almost all the energy required to deform sample is elastically recoverable. Therefore, we will consider -J400 as an elastic material (instead of viscoelastic) at ambient temperature, with a Young’s modulus equal to the storage modulus. This however is not the case for the other two materials: their viscoelastic behavior makes it impossible to neglect the viscous contribution at 25 °C and attention should be given to this in further applications. To derive the fracture stress and strain, a static stress-strain test until fracture was carried out in tension.
Mixing ability
The studied polymers differ only in spacer length, which makes it possible to mix furan compounds having a different degree of polymerization, during the synthesis of the SHmaterial. Using this mixing method a DA-polymer is obtained with a mixture of spacer lengths. In this way a polymer with desirable, intermediate material properties lying in the broad interval between the two extremes (J400 and J4000) can be obtained. This mixing ability is a great advantage since it provides a certain degree of freedom in the design of future self-healing actuator applications.
Synthese of Diels-Alder polymers
Reagens
The reactants, the monomers, used in the synthesis of the reversible DA-polymer network system were purchased from Sigma-Aldrich:
- (A): 2 Jeamine D-series: Poly(propylene glycol) bis(2-aminopropyl ether) with average degree of polymerization n (determined by nuclear magnetic resonance spectroscopy):
- n = 44.2: J2000, Mn = 2640 g/mol
- n = 71.1: J4000, Mn = 4200 g/mol
- (B): furfuryl glycidyl ether (FGE, 96 %)
- (C): 1,1'-(methylenedi-1,4-phenylene)bismaleimide (DPBM, 95%)
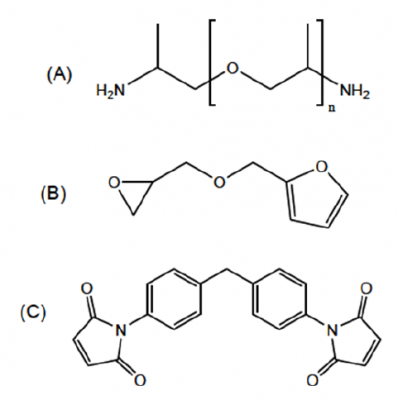
Polymerization
In this section it is explained how the DA-polymers are synthesized. J4000 and J2000 material is synthesized into sheets while J400 is synthesized into powder. For each step the chemical reaction is explained followed by a description of the practical actions required to preform this step. The following amounts of DA-polymer material was made:
- DPBM-FGE-J4000: 100x100 mm sheet with a 0.75 mm thickness.
- DPBM-FGE-J2000: 100x100 mm sheet with a 0.50 mm thickness.
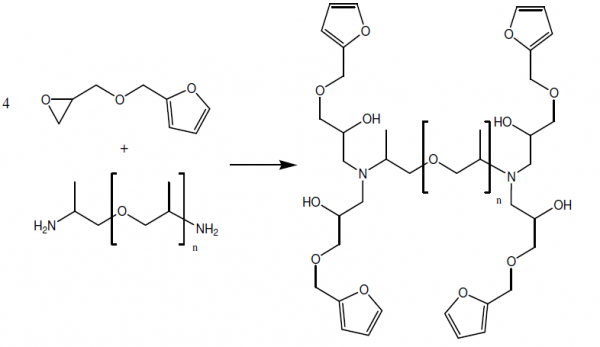
Step 1: epoxy-amine reaction
In a first step, FGE was irreversibly bonded to Jx (x = 400, 2000, and 4000) through an epoxy-amine reaction, yielding a furan functionalized compound. This reaction was performed at 60 °C for minimum 7 days after which the reaction was completed at 90 C° (to speed up the reaction) for 2 days.
In practice: Pour the FGE and the Jeffamine together. The following amounts were used:
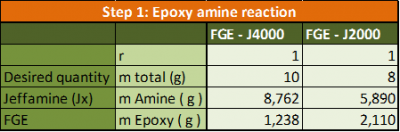
- Heat the solution using an oil bath to 60°C for minimum 7 days. Stir continuously using a magnetic stirrer.
- Increase the temperature to 90°C for another 2 days.

Step 2: Network formation
In a second step, the furan functionalized compound (FGE-Jx) was mixed with DPBM in a stoichiometric ratio of one (r= nMaleimide / nFuran = 1) to obtain the reversible polymer network through DA reaction. Prior to the DA reaction, the DPBM was dissolved in chloroform (a 20 w% solution) to obtain a homogeneous reversible network.
In practice: Pour the FGE and the Jeffamine together. The following amounts were used:
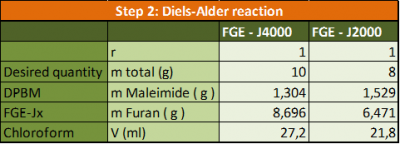
- Pour the chloroform at the FGE-Jx
- Add the DPBM to the solution
- Stir at room temperature for at least 2 hours, using a magnetic stirrer, until a completely homogeneous solution is obtained

Step 3
The chloroform has to be evaporated out of the solution and the thermo-reversible network has to be formed. The chloroform is extracted by increasing the temperature to 60°C at vacuum. The thermo-reversible network is formed by slowly cooling down the sheet after the chloroform has been completely extracted.
In practice: The following amounts were used to develop the DPBM-FGE-Jx sheets:
- DPBM-FGE-J4000: 28.1 ml
- DPBM-FGE-J2000: 18.8 ml
Step by Step:
- 1 hour at 30°C and atmospheric pressure
- gradually increase the temperature up to 660°C
- gradually decrease the pressure up to vacuum.
- remain at 60°C and vacuum until all bells are removed
- remain for 3 days at 50°C and vacuum
- cool down slowly (5°C/hour) to ambient temperature after which the synthesis is completed

Design
Conceptual design of the SH-soft pneumatic actuator
Most SPAs, like the BSPA, consist of more cells, containing air chambers. To evaluate the potential of creating a SH-SPA, a single soft pneumatic cell (SPC), built entirely out of theDPBM-FGE-Jx material, is designed. The SPC is a cubic cell with a side length of 15mm. It is assumed that starting from this single-cell prototype, it is straightforward to build a multi-cell prototype, a (Bending) SPA. The cubic cell was developed out of the most flexible, -J4000 material (storage modulus: 7.85 MPa). -J4000 is less soft in comparison with Ecoflex 00–30 (tensile modulus: 69 kPa) usually used. However, due to the high fracture strain of 450%, the SPC can be used to create relative large deformations before a failure occurs. If multiple self-healing soft pneumatic cells (SH-SPCs) would be put in series, a BSPA could be constructed.
The -J2000 (storage modulus: 28 MPa) material is less flexible than the -J4000 material and is therefore suitable to be used to create the anisotropy needed in the SPC design. Based on the design of the BSPA, the bottom sheet was produced out of the less flexible material, -J2000, in order to create an anisotropic movement response.
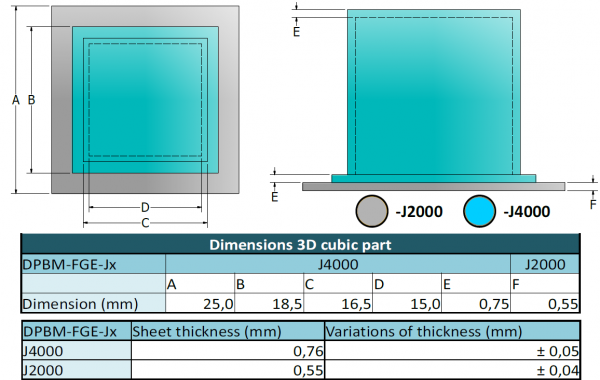
The dimensions of the SH-SPC are presented in the figure below. Because the -J4000 and -J2000 sheets were synthesized through solvent casting, the sheet thicknesses have a small variation. Combining the different mechanical properties of -J2000 and -J4000, a fully SH-SPC is developed, in which the air chamber as well as the less-stretchable sheet have the SH-property. In the bottom of the SPC, a small hole is made in which a narrow metal tube is inserted through which the over-pressured air can enter the cell.
Manufacturing
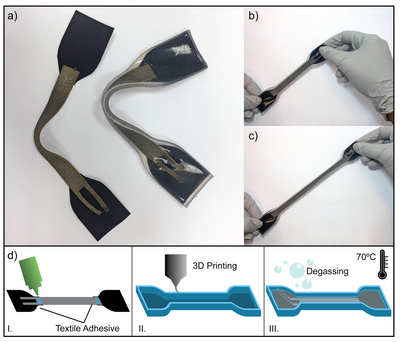
Shaping through folding and self-healing
We present a completely new shaping method to develop 3D polygon structures starting from synthesized SH-polymers sheets, which utilizes the SH-properties of these polymers. This shaping process is termed, "shaping through folding and self-healing". In this process, a 3D polygon structure is developed by folding a 2D SH-polymer sheet, similar to making an origami structure. The sides of 3D polygon structure are made air tight using a SH-procedure at relative low temperatures, not higher than 90°C.
The construction of the cubic SPC entails three SH-procedures. The temperature profiles (T-profile) of these procedures differ: the temperature and the duration of the isothermal step depend on the required viscosity for sealing the 3D structure. For these three only the T-profiles will be given in, we will not discuss in detail the behavior of the polymer network during these procedures.

Construction of the SPC
Step 1
First, a plus shape was cut out of the synthesized DPBM-FGE-J4000 sheet. This plus sign was folded and placed into the Teflon Mold. Teflon is used because of its chemical inertness and because it prevents the polymer from sticking to the mold, which would happen when glass or metal molds are used. To seal the sides of the open cube, the SH-property of the material was used.
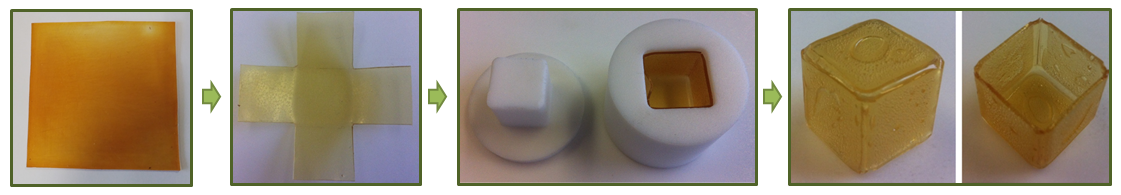
The mold was placed in a furnace, at a temperature of 3 °C below the gel transition temperature (Tgel = 81.0 °C). At this elevated temperature the polymer chains have enough mobility to close the gaps between the vertical planes of the cube in a few hours. The temperature cannot exceed the Tgel, because the sheets inserted in the mold would start deforming. The SH-process used to form the DPBM-FGE-J4000 (open) cube, was done in a furnace at 78 °C for 4 hours. After this the part was cooled down with a gradient of ± 0.5 C ◦ min −1. Hereafter, the cubic part remained for 24 hours in the mold at 25 °C such that the conversion could converge to the equilibrium conversion. The cubic part was gently removed from the cubic mold.
Step 2
Next, a stiffer sheet is connected to the open side of the cubic part. This bottom part consists of a -J4000 sheet that was placed directly on the cubic part and a stiffer bottom -J2000 sheet. The additional -J4000 sheet placed between the cubic part and the -J2000 sheet was used to create a better connection. The Tgel of the -J2000 series (98.5 °C) is higher than the one of -J4000 (81.0 °C). The -J4000 sheet was placed on top of the J2000 sheet, and this was placed in a furnace at 90 °C. After 15 min at this high temperature, the top -J4000 sheet turned into a gel-like structure, while the -J2000 sheet remained solid. At that time, the sheets were taken out of the furnace and the cold -J4000 cubic part was immediately pushed on top of the gel-like -J4000 sheet, which sealed the cubic part. The furnace was brought to 60 °C before the whole part was reinserted. The furnace was held at this temperature for 6 hours after which it was cooled down to room temperature at 0.5 °C min −1. Before the shaping process continued the part was left untouched for 24 hours, to reach equilibrium conversion.
 Step 3
Step 3
Finally a small metal tube had to be inserted in the bottom plate of the SPC, through which the compressed air will be injected in the cell. A hole (D = 2 mm) was made in the bottom sheet of the cubic part. Two small pieces (5 × 5 mm) were cut out of the -J4000 sheet and placed over the metal tube. Next this metal piece was placed in a furnace at 90 °C. Again after 15 min, the -J4000 polymer pieces had attained a gel-like behavior and the metal piece was taken out of the furnace. The hot metal tube was pressed through the hole in the cubic bottom part and the gel-like -J4000 pieces sealed the connection. The furnace was cooled down to 60 °C, after which the assembly was reinserted, held at this temperature for 4 hours, and finally cooled down naturally in the furnace to room temperature.
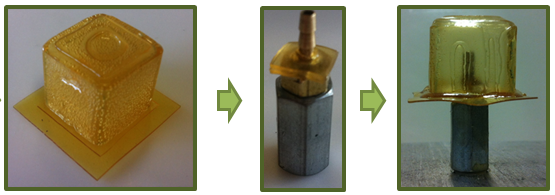
The SPC-prototype resulting from the manufacturing was constructed out of 2D DA-polymer sheets, using three SH-procedures. For this manufacturing technique, which relies on shaping through folding and self-healing, only relatively low temperatures are required (Tmax = 90 °C).
Experimental Validation
Mechanical properties
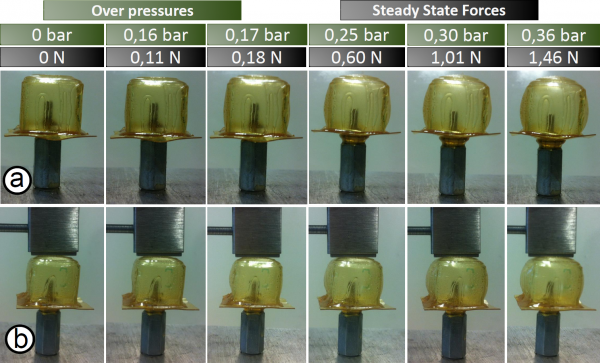
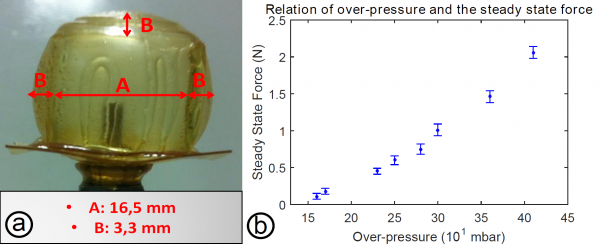
The developed SH-SPC was experimentally tested for different over-pressures of the air chamber. The resulting deformations of the SPC were captured using a digital camera. The cell was tested for over-pressures starting from 0 bar up to 0.36 bar. A zoomed image is presented, showing the deformation of the SPC for an over-pressure of 0.36 bar. For this over-pressure the width of the cubic cell increases with 40%.
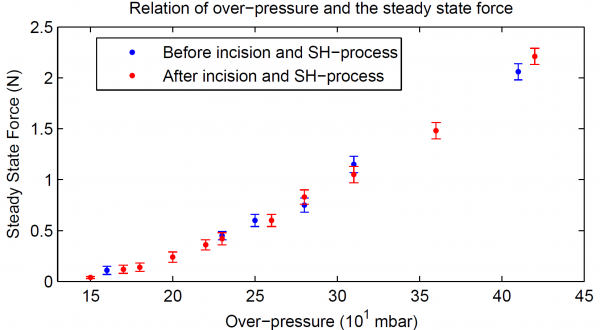
The force exerted by the top plane of the SH-SPC was measured using a force sensor. These are steady state forces, the constant force applied by the SH-SPC on the force sensor over a long time. The steady state force is represented as a function of over-pressure. The forces are in the range of a few newtons and are, compared to values measured in literature, suitable for SPA applications.
The combination of forces in the range of newtons that can be applied by the SH-SPC and the high deformation response for over-pressures lower than 0.5 bar, indicate that the mechanical properties of the DA-materials, DPBM-FGE-J4000 and -J2000, are adequate for small actuation in soft robotic.
Self-healing properties
After the validation of the mechanical properties of the SH-SPC, the cell was pushed to its limits: the over-pressure was increased until a small perforation occurred in the cell at a maximum over-pressure of 0.46 bar. The location of the perforation is presented in figure below. At this location the open cube, created entirely out of -J4000 material, is connected to the -J2000 sheet. The -J4000 is more elastic and when the air chamber is put under overpressure, the cubic cell starts to deform. -J2000 on the other hand, is less elastic and will only deform only a little. This causes stress concentrations at the connection between the two polymer materials. The perforation of the SH-SPC took place on the location where failure was theoretically expected. From this it can be concluded that the sides of the 3D cubic cell were well-sealed in the manufacturing process, using the new developed technique: "shaping through folding and self-healing".
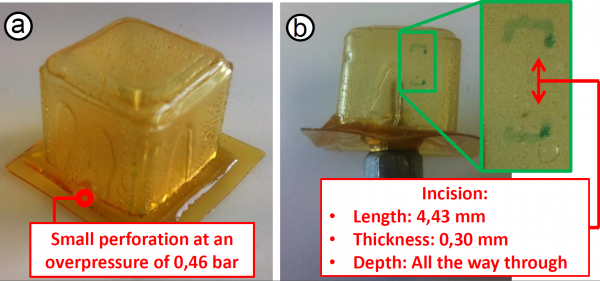
To evaluate the SH-property of the SPC, an incision was made with a scalpel in one of the planes of the cubic part. The dimensions of this
incision are: a length of 4.43 mm, a thickness of 0.30mm (blade) and all the way through the wall of the cell. Subsequently this incision as well as the perforation were self-healed in an external furnace using the SH-procedure. This procedure had a maximum temperature of 70 °C and a duration of 30 hours.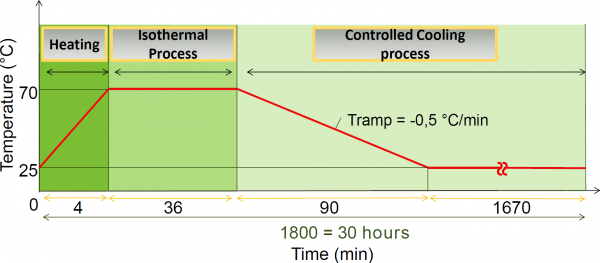
In order to determine whether the initial mechanical properties of the SH-SPC were recovered after the SH-procedure, the steady state force was again measured as a function of the over-pressure. The results of this test were plotted in figure Below, together with the data measured before the incision was made. As the results for the two experiments are the same within the scatter of the data, the SH-SPC has the same mechanical
performance before and after the SH-procedure. After these experiments, the SH-SPC was again pushed to its limits. A perforation took place at 0.48 bar, at the same location it did before. This indicates that the incision was completely cured and no weak spots were created during and after the SH-procedure. In addition, comparing the maximal over-pressure before (0.46 bar) and after (0.48 bar) the SH-procedure, it can be concluded that the puncture, created before the incision was made, was also entirely healed.